
Healsee has an internationally advanced automatic production line, which can realize the automation of the whole process from sol to production to inspection to storage, effectively improving the production efficiency and ensuring the consistency and stability of product quality.
Healsee capsules have passed the technical review of the NMPA (“A” for registered status), KOSHER certification, HALAL certification, BRC certification, NSF certification, "Quality, environment, occupational health and safety" management system certification (ISO) and energy management system certification. The product meets the needs of customers in major countries and regions around the world.
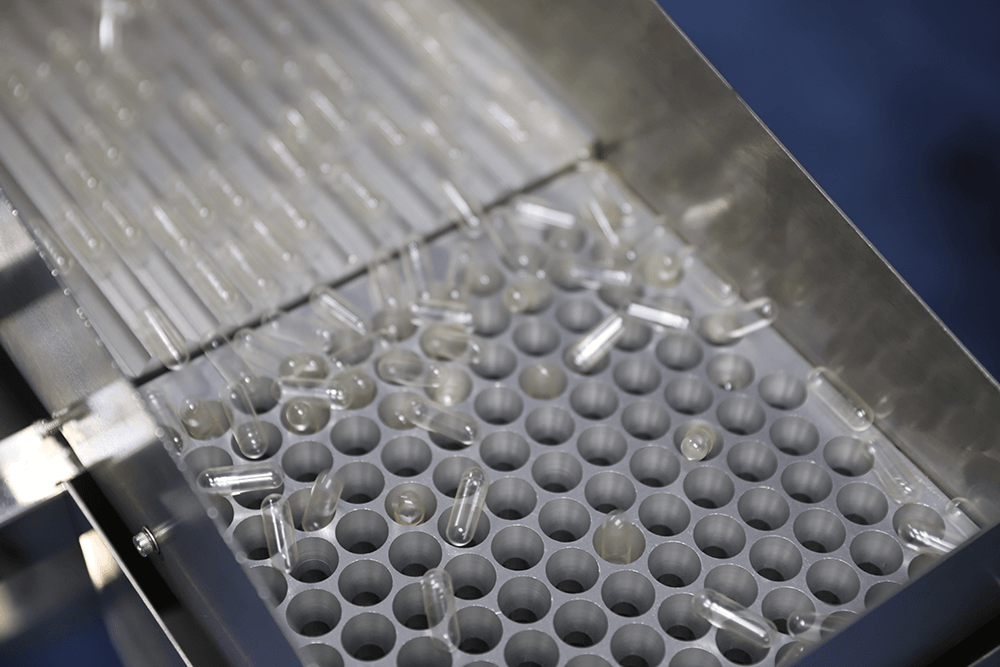
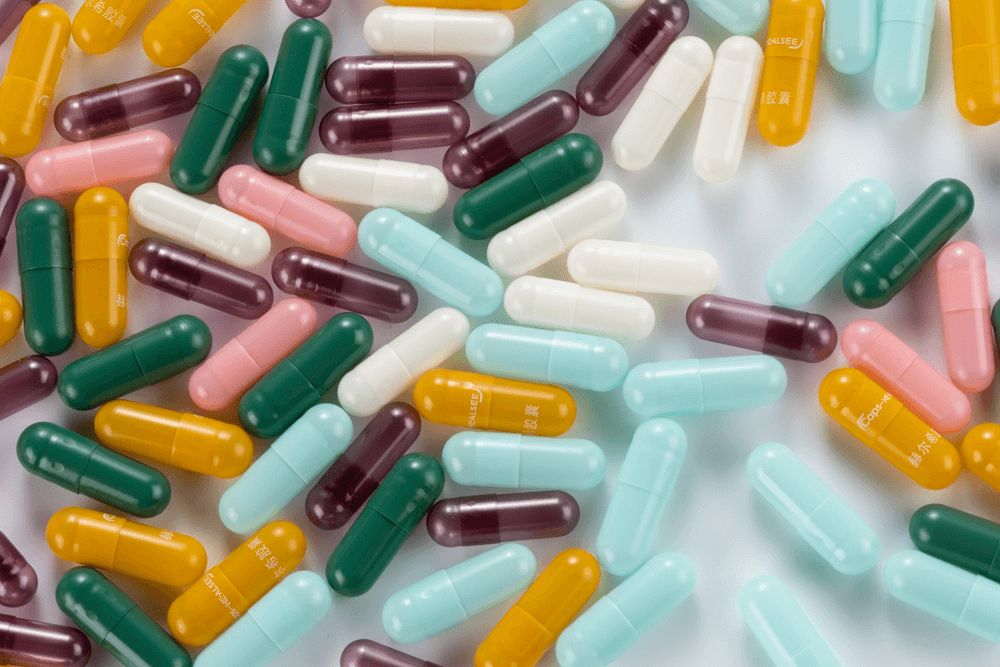
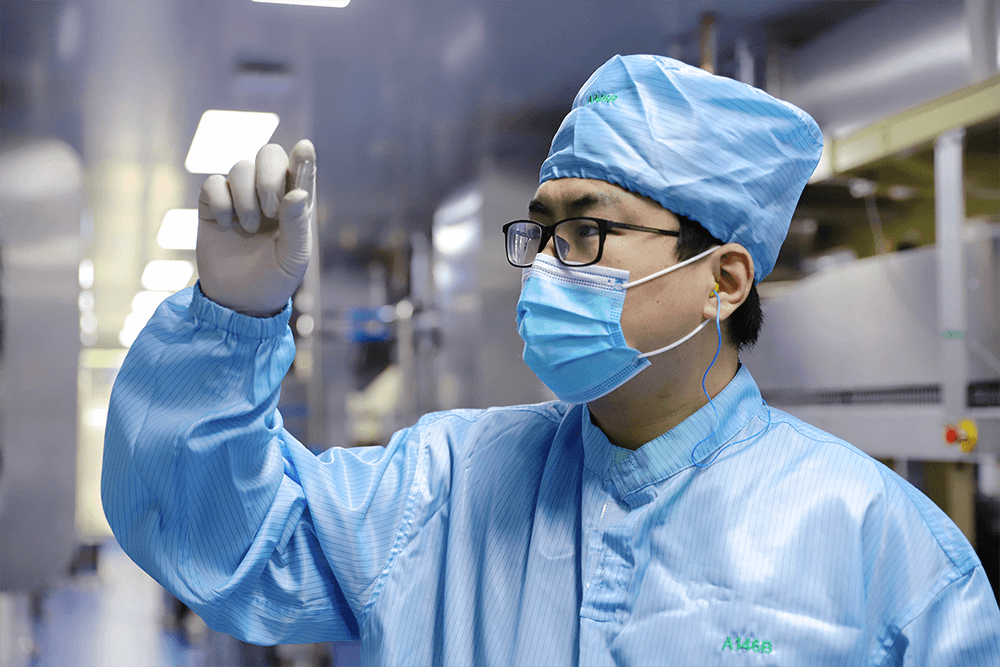
HPMCHPMC

The raw materials are sourced from HPMC produced by the parent company, Head Group. Derived from natural wood fiber, they are GMO-free and complies with GMP standards, which are safe and environmentally friendly, effectively avoiding the potential risk of animal-derived diseases.
Leveraging the integrated advantages of the Group’s upstream and downstream industrial chain, Healsee is able to maximize the synergistic effect of product research and development. With its robust R&D and innovation capabilities, it ensures a multi-faceted safeguarding of raw materials, innovation, and quality, catering to a variety of customer needs.
Each batch of raw materials is strictly inspected in accordance with the enterprise quality standards formulated by the national pharmacopoeia in China to ensure stable and safe raw material quality.